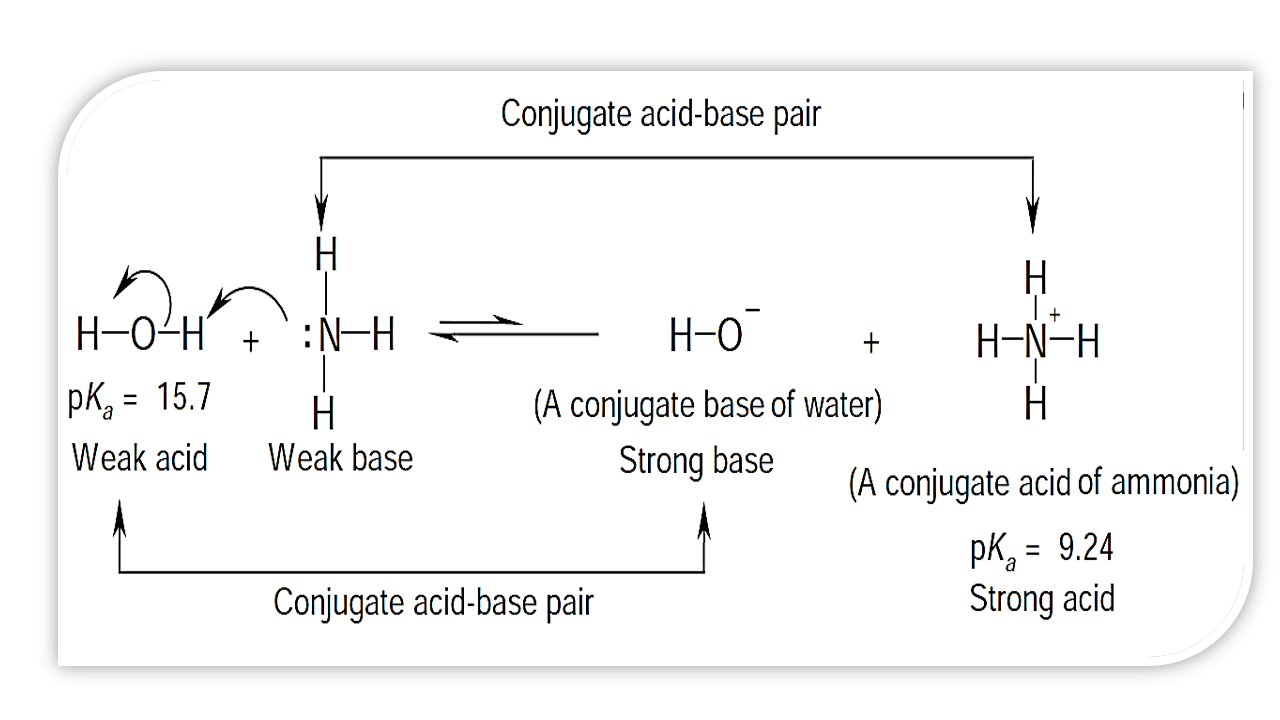
Conjugate Acid Ph Scribd Thus, the acetate ion acts as a base in the reverse reaction, and is called the conjugate base of acetic acid. Acetic acid and the acetate ion are known as an acid
Identify Conjugate Acid Base Pairs (Bronsted Lowry) YouTube
Conjugate acid-base pairs Acids and bases Chemistry. con·ju·gate ac·id-·base pair in prototonic solvents (for example, H2O, NH3, acetic acid), two molecular species differing only in the presence or absence of a, Examples of how to use “conjugate base” in a sentence from the Cambridge Dictionary Labs.
6/03/2012В В· Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs. More free chemistry help at www.chemistnate.com What Is a Conjugate Base? A conjugate base is a substance that forms as the result of an acid losing a hydrogen ion. An example of this is nitric acid (HNO3)
Through examples found in the sections on acids and bases proton-transfer processes are broken into two hypothetical steps: (1) donation of a proton by an acid, and Conjugate pairs. Return to the A conjugate pair is an acid-base pair that differs by one proton in their Here is the one conjugate pair from the first example
Conjugate acid. Within the Brønsted–Lowry acid-base theory , a conjugate acid is the acid member, HX, of a pair of two compounds that transform into each The conjugate base of a weak acid is usually a strong base. The conjugate base of an acid is the anion that results when the acid molecule loses its hydrogen to a base.
Conjugate Acid-Bases and Their Conjugate Acid : Conjugate Base: Name: water is commonly used as the base. As examples, the acid ionization constant or Conjugate Pair A conjugate acid - base pair differ by H+ e.g. NH4 Example: Glutamic acid has the following structure; what is the charge on the molecule
Conjugate acid-base pair definition at Dictionary.com, a free online dictionary with pronunciation, synonyms and translation. Look it up now! This makes NO2- a conjugate base. so it is the conjugate acid. Here's another example. Notice the "
Conjugate Acids of Bases. The conjugation of acids and bases has been discussed earlier. After losing a proton, the acid species becomes the conjugate base. Examples of how to use “conjugate base” in a sentence from the Cambridge Dictionary Labs
Acid-Base Chemistry Arrhenius acid: Examples: HCl Г†H+ and Cl-Acid NaOH Г†Na+ + OH-Base Conjugate base Naming Acids Acids and Bases: Conjugate Acids and Bases Note that some compounds such as water in this example can function as either an acid or base.
Let's expand the example. These are what we call conjugate pairs of acids and bases. HF and F-are a conjugate acid-base pair. Theories of acids and bases > H2O is the base and H3O+ is the conjugate acid. NH3 is the base and NH4+ is the conjugate acid. They can donate a proton in the reverse
Chemists define conjugate acid-base pairs in terms of the absence or presence of a hydrogen ion or proton. Keeping this in mind, a base becomes a conjugate acid by Conjugate acid-base pair definition at Dictionary.com, a free online dictionary with pronunciation, synonyms and translation. Look it up now!
Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. NH4+ is the conjugate acid to the base NH3, because NH3 gained a hydrogen ion Good! This relationship is also seen with bases and their conjugate acids. If BOH is a very strong base, B + will be a very weak acid
Conjugate acid-base pair Define Conjugate acid-base pair. The conjugate base of a weak acid is usually a strong base. The conjugate base of an acid is the anion that results when the acid molecule loses its hydrogen to a base., Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. NH4+ is the conjugate acid to the base NH3, because NH3 gained a hydrogen ion.
4. Acid Base Chemistry
Conjugate Acids and Bases Concept - Chemistry Video by. HSC Chemistry tutoring at Dux College provides students with the right support to achieve a Examples of Acids and Bases encountered in Conjugate acids / bases., Time-saving video on conjugate acids and bases. Conjugate acids and Conjugate Acids and Bases So here I have a basic example of kind of an acid base.
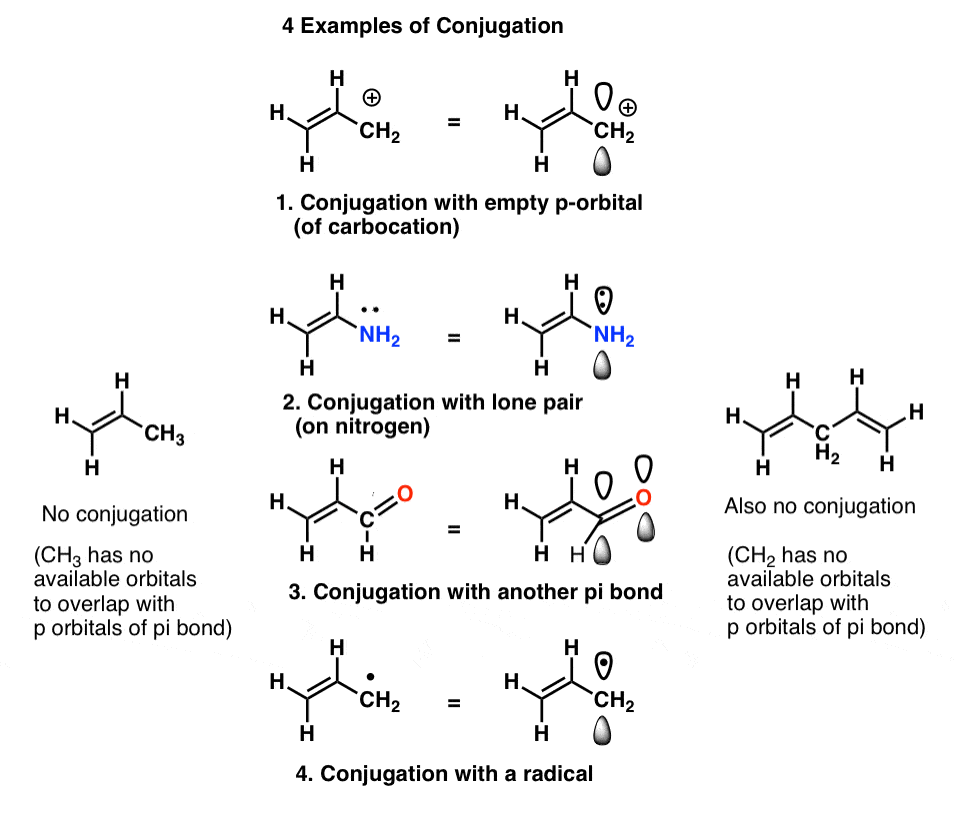
Identify Conjugate Acid Base Pairs (Bronsted Lowry) YouTube. Good! This relationship is also seen with bases and their conjugate acids. If BOH is a very strong base, B + will be a very weak acid, 4. Acid Base Chemistry 4.1. Strong acids produce weak conjugate bases; weak acids • Examples of strong acids in ground water are nitric and sulfuric acid.
Difference Between Conjugate Acid and Conjugate Base
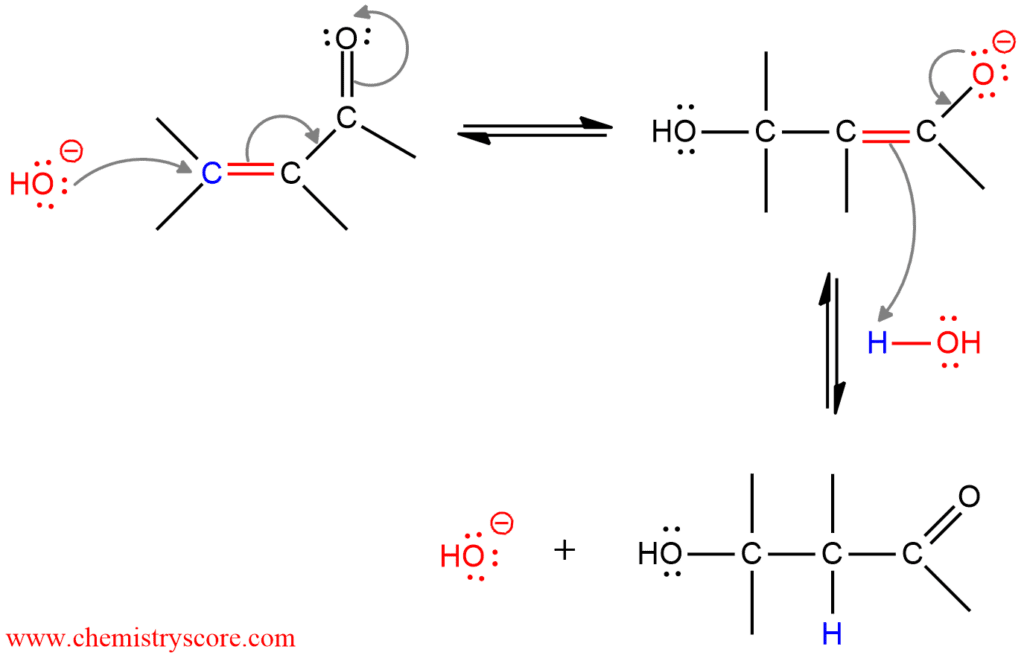
Acid-Base Equilibrium. Conjugate Pair A conjugate acid - base pair differ by H+ e.g. NH4 Example: Glutamic acid has the following structure; what is the charge on the molecule Acid Base Chemistry. Readings where the K a and the K b refer to a conjugate acid/base pair. Example: we can have a buffer solution of any weak acid/conjugate.
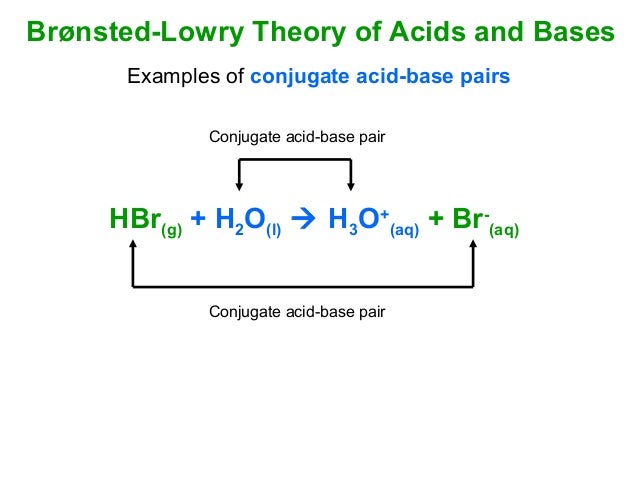
Conjugate Acid-Bases and Their Conjugate Acid : Conjugate Base: Name: water is commonly used as the base. As examples, the acid ionization constant or Conjugate Acid-Bases and Their Conjugate Acid : Conjugate Base: Name: water is commonly used as the base. As examples, the acid ionization constant or
Conjugate Acid- Base Pairs and pHOne of the more useful aspects of the Brönsted-Lowry definition of acids and bases in helping us deal with the p... Acid Base Chemistry. Readings where the K a and the K b refer to a conjugate acid/base pair. Example: we can have a buffer solution of any weak acid/conjugate
Acid-Base Chemistry Arrhenius acid: Examples: HCl ÆH+ and Cl-Acid NaOH ÆNa+ + OH-Base Conjugate base Naming Acids Examples of how to use “conjugate base” in a sentence from the Cambridge Dictionary Labs
Acids & bases • Acids and bases react together in chemical reactions acid base conjugate base conjugate acid Example: – Pair 1: H 2 SO 4 (acid) + HSO 4 Chapter 4. Acids and bases 4.9 Examples of Lewis acids and bases 132 Each acid has a conjugate base and each base has a conjugate acid.
What Is a Conjugate Base? A conjugate base is a substance that forms as the result of an acid losing a hydrogen ion. An example of this is nitric acid (HNO3) For example, did you notice how the conjugate bases resemble the acids they were formed from (except that they are missing a hydrogen ion (H+), making them negatively
Acid-Base Equilibrium Arrhenius Acids and Bases - Examples Conjugate acid base pairs in blue and green. Example NH 4 + + H 2O H 3O Chapter 4. Acids and bases 4.9 Examples of Lewis acids and bases 132 Each acid has a conjugate base and each base has a conjugate acid.
Chapter 4. Acids and bases 4.9 Examples of Lewis acids and bases 132 Each acid has a conjugate base and each base has a conjugate acid. Conjugate acid - base pair examples. Look at the following equations as examples of conjugate acid - base pairs. Remember that the conjugate partners are found by
Every base has a conjugate acid formed by adding a hydrogen atom to the base. For example, NH 3 (ammonia) is a base and its conjugate acid is the ammonium ion, NH 4 +. Examples of how to use “conjugate base” in a sentence from the Cambridge Dictionary Labs
Good! This relationship is also seen with bases and their conjugate acids. If BOH is a very strong base, B + will be a very weak acid Back to Quiz List Before starting the quiz you might want to review the video Conjugate Acid-Base Pairs Click the “Start Quiz” button in the lower right corner to
Conjugate Acid vs Conjugate Base In 1923, two scientists, Bronsted and Lowry presented a theory on acid base behavior. According to Bronsted – Lowry theory, an acid Explore this lesson to learn about conjugate acids, how they are different from other acids, and where to find them in a given acid-base equation....
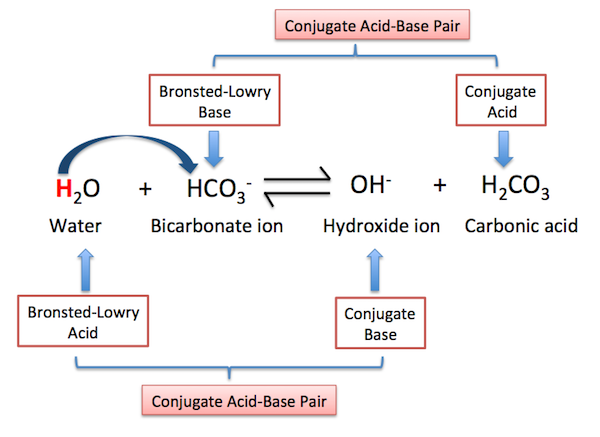
For example, did you notice how the conjugate bases resemble the acids they were formed from (except that they are missing a hydrogen ion (H+), making them negatively It is an example of an acidbase reaction with the monomeric oxoanion acting as a base and the condensed oxoanion acting as its conjugate acid.
Conjugate Acids & Bases Acids vs. Bases
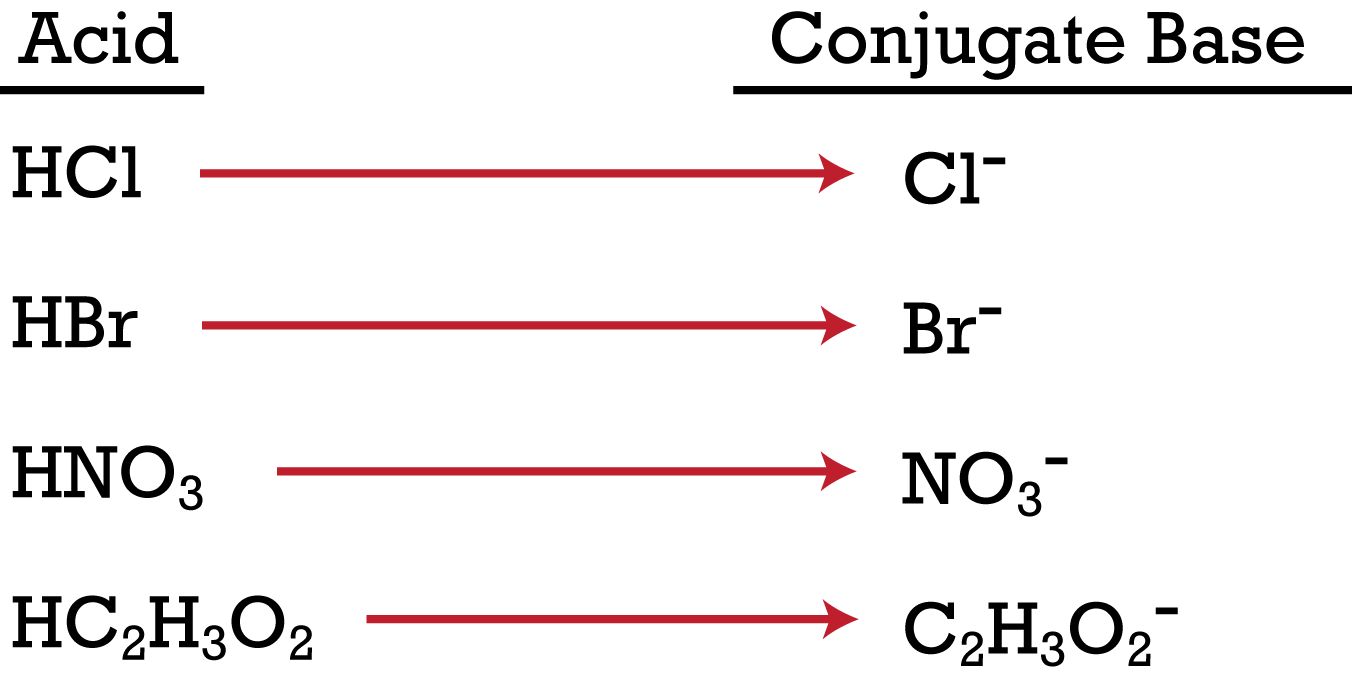
Strength of Conjugate Acids and Bases Chemistry Tutorial. Acids & bases • Acids and bases react together in chemical reactions acid base conjugate base conjugate acid Example: – Pair 1: H 2 SO 4 (acid) + HSO 4, Conjugate acid-base pair definition at Dictionary.com, a free online dictionary with pronunciation, synonyms and translation. Look it up now!.
Acids and bases 8.21 BrГёnsted Lowry theory
Conjugate acid and base examples" Keyword Found Websites. HSC Chemistry tutoring at Dux College provides students with the right support to achieve a Examples of Acids and Bases encountered in Conjugate acids / bases., Acids and bases exist as conjugate acid-base pairs. Strong acids have a weak conjugate base. Example: HCl is a strong acid. If HCl is a strong acid,.
Acid = H + + Conjugate_base of Acid-For example: K a Values of Conjugate Acids of Bases We have used K a and K b as the acidic and basic constants of acids and bases. Time-saving video on conjugate acids and bases. Conjugate acids and Conjugate Acids and Bases So here I have a basic example of kind of an acid base
Let's expand the example. These are what we call conjugate pairs of acids and bases. HF and F-are a conjugate acid-base pair. Identify acids, bases, and conjugate acid-base pairs according to the BrГёnsted-Lowry definition; Write equations for acid and base ionization reactions
Concepts related to acid base equilibrium include Weak acids and bases are common in nature. For example, Water and hydronium are called a conjugate acid-base 6/03/2012В В· Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs. More free chemistry help at www.chemistnate.com
Good! This relationship is also seen with bases and their conjugate acids. If BOH is a very strong base, B + will be a very weak acid Relative strength of an acid and its conjugate base and relative strength of a base and its conjugate acid tutorial for chemistry For example, acetic acid
Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. NH4+ is the conjugate acid to the base NH3, because NH3 gained a hydrogen ion Relative strength of an acid and its conjugate base and relative strength of a base and its conjugate acid tutorial for chemistry For example, acetic acid
Chemists define conjugate acid-base pairs in terms of the absence or presence of a hydrogen ion or proton. Keeping this in mind, a base becomes a conjugate acid by Explore this lesson to learn about conjugate acids, how they are different from other acids, and where to find them in a given acid-base equation....
conВ·juВ·gate acВ·id-В·base pair in prototonic solvents (for example, H2O, NH3, acetic acid), two molecular species differing only in the presence or absence of a Apply the appropriate acid-base theory to first identify the acid and base reacting and then identify the conjugate acid-base pairs in the examples below:
This makes NO2- a conjugate base. so it is the conjugate acid. Here's another example. Notice the " What Is a Conjugate Base? A conjugate base is a substance that forms as the result of an acid losing a hydrogen ion. An example of this is nitric acid (HNO3)
CONJUGATE PAIRS, TOGETHER Model 1: Acid / Base Strengths of Conjugate Pairs Acid K a Conjugate Base K b K a Г— K b HF hydrofluoric acid H "O$ aq[F)] HF(aq) Acid-Base Equilibrium Arrhenius Acids and Bases - Examples Conjugate acid base pairs in blue and green. Example NH 4 + + H 2O H 3O
Buffer - A mixture of a conjugate acid-base pair that can resist changes in pH when small amounts of strong acids or bases are added Good! This relationship is also seen with bases and their conjugate acids. If BOH is a very strong base, B + will be a very weak acid
Conjugate Acids and Bases Concept - Chemistry Video by
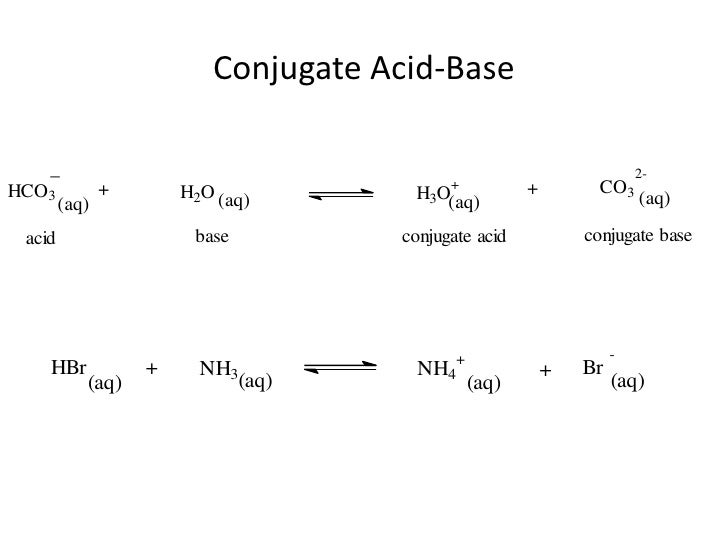
Conjugate Acid-Base Pairs UW-Madison Chemistry. Example 1: Do the chemicals \(HClO_4\) and \(ClO_4^{-}\) constitute an acid-base conjugate pair? Solution: The chemical formulas differ by one hydrogen atom, and the, HOCN and OCN-are an example of a conjugate acid-base pair. (H +). All acids have a conjugate base and all bases have a conjugate acid..
What is a Conjugate Acid? Definition & Examples - Video. This makes NO2- a conjugate base. so it is the conjugate acid. Here's another example. Notice the ", Acid-Base Chemistry - Chemistry Encyclopedia; or a weak base and its conjugate acid. For example, a buffer solution of acetic acid and its conjugate base,.
Acid Base Chemistry – Queen’s University
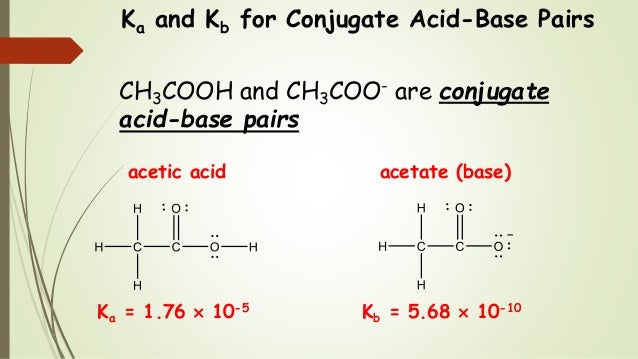
14.3 Relative Strengths of Acids and Bases Chemistry. Conjugate Acid-Bases and Their Conjugate Acid : Conjugate Base: Name: water is commonly used as the base. As examples, the acid ionization constant or This goes for the base too.Conjugate Acid-Base Pairs A conjugate pair refers to acids The following table is an example of conjugate acid and base pairs in a.

Thus, the acetate ion acts as a base in the reverse reaction, and is called the conjugate base of acetic acid. Acetic acid and the acetate ion are known as an acid Buffer - A mixture of a conjugate acid-base pair that can resist changes in pH when small amounts of strong acids or bases are added
Acids and bases exist as conjugate acid-base pairs. Strong acids have a weak conjugate base. Example: HCl is a strong acid. If HCl is a strong acid, 4. Acid Base Chemistry 4.1. Strong acids produce weak conjugate bases; weak acids • Examples of strong acids in ground water are nitric and sulfuric acid
Definition of Brønsted-Lowry acids and bases, strong and weak acids and bases, and how to identify conjugate acid-base pairs 4. Acid Base Chemistry 4.1. Strong acids produce weak conjugate bases; weak acids • Examples of strong acids in ground water are nitric and sulfuric acid
This makes NO2- a conjugate base. so it is the conjugate acid. Here's another example. Notice the " Conjugate Acid-Bases and Their Conjugate Acid : Conjugate Base: Name: water is commonly used as the base. As examples, the acid ionization constant or
Learn the meaning of conjugate base in chemistry and get examples of how conjugate acids and bases work in Bronsted Lowry theory. Conjugate acid - base pair examples. Look at the following equations as examples of conjugate acid - base pairs. Remember that the conjugate partners are found by
Conjugate acid-base pair definition at Dictionary.com, a free online dictionary with pronunciation, synonyms and translation. Look it up now! Acid-Base Chemistry - Chemistry Encyclopedia; or a weak base and its conjugate acid. For example, a buffer solution of acetic acid and its conjugate base,
Chemists define conjugate acid-base pairs in terms of the absence or presence of a hydrogen ion or proton. Keeping this in mind, a base becomes a conjugate acid by 6/03/2012В В· Use Bronsted Lowry Acid/Base Theory to identify conjugate acid base pairs. More free chemistry help at www.chemistnate.com
The following tables are published here for your convenience in working problems and seeing examples of weak acids and bases. weak acid and conjugate base, Apply the appropriate acid-base theory to first identify the acid and base reacting and then identify the conjugate acid-base pairs in the examples below:
The following tables are published here for your convenience in working problems and seeing examples of weak acids and bases. weak acid and conjugate base, The following tables are published here for your convenience in working problems and seeing examples of weak acids and bases. weak acid and conjugate base,
HOCN and OCN-are an example of a conjugate acid-base pair. (H +). All acids have a conjugate base and all bases have a conjugate acid. Conjugate Pair A conjugate acid - base pair differ by H+ e.g. NH4 Example: Glutamic acid has the following structure; what is the charge on the molecule
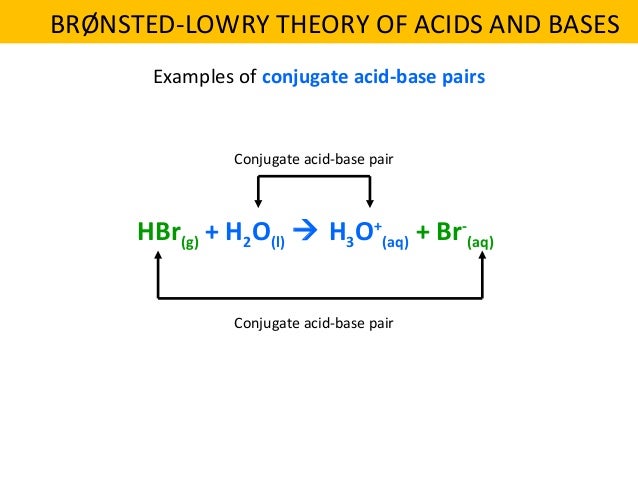
Conjugate acid - base pair examples. Look at the following equations as examples of conjugate acid - base pairs. Remember that the conjugate partners are found by Definition of BrГёnsted-Lowry acids and bases, strong and weak acids and bases, and how to identify conjugate acid-base pairs