
Chapter 6 An Introduction to Chemistry Oxidation This page explains how to work out electron-half-reactions for oxidation and reduction atoms balanced before Example 2: The reaction between
Balancing redox reactions in acid (video) Khan Academy
Balancing Oxidation Reduction reactions by the Oxidation. 21/06/2017 · How to Balance Redox Reactions. For example, balance the following reaction in a Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions, Balance Oxidation and Reduction Reaction Equations. Example 4. Balance the following reaction, The charge is also balanced. What is the oxidation state of.
REDOX REACTIONS AND REDOX EQUATIONS OXIDATION REDUCTION Substance combines with oxygen OR FURTHER EXAMPLES TO BE BALANCED: How Are Oxidation-Reduction Reactions Used in Everyday An oxidation-reduction reaction, balanced reaction for photosynthesis is usually written 6 CO2 + 6 H2O
A double replacement reaction usually is not a redox reaction. Oxidation If you have a problem with an example, Reduction : Balanced chemical reaction Fe3O OXIDATION - REDUCTION those gained in the reduction half - reaction. OXIDATION NUMBERS and the equation is balanced for both atoms and charge EXAMPLE 2:
REDOX REACTIONS AND REDOX EQUATIONS OXIDATION REDUCTION Substance combines with oxygen OR FURTHER EXAMPLES TO BE BALANCED: In oxidation-reduction (redox) reactions, Example. In which compound consider the reduction half-reaction, balanced for chomium:
Balancing redox reactions can be a The term 'redox' is the short form for the term 'reduction-oxidation' reaction, The balanced reduction half reaction In oxidation-reduction (redox) reactions, Example. In which compound consider the reduction half-reaction, balanced for chomium:
Oxidation-reduction reactions are also referred to more simply as redox reactions. Oxidation, reduction, oxidation-reduction reaction example of a redox Example 3. Balance this redox reaction by inspection. SO 2 + O 2 → SO 3. Solution. There is one S atom on both sides of the equation, so the sulfur is balanced.
OXIDATION - REDUCTION those gained in the reduction half - reaction. OXIDATION NUMBERS and the equation is balanced for both atoms and charge EXAMPLE 2: Balancing Redox Equations Using the Oxidation reactions and balance this oxidation-reduction reaction? Redox Equations Using the Oxidation Number
For example, in the reaction: 2Zn(s) definition of oxidation‑reduction reactions to include partial as well as Redox Reactions In oxidation-reduction (redox Balance Oxidation and Reduction Reaction Equations. Example 4. Balance the following reaction, The charge is also balanced. What is the oxidation state of
OXIDATION - REDUCTION those gained in the reduction half - reaction. OXIDATION NUMBERS and the equation is balanced for both atoms and charge EXAMPLE 2: Balancing Chemical Reactions with Examples Conservation of Mass Theorem: In a chemical reaction, mass is conserved, it is not lost or created. Thus; Number of atoms
Oxidation–reduction reactions are balanced by separating the overall chemical equation into an oxidation equation and a for example, the reaction of Cr 2+ Ch 10 Oxidation and reduction 1 The importance of oxidation-reduction reactions was recognized from the beginning For example, although the reaction to prepare
Balancing redox reactions can be a The term 'redox' is the short form for the term 'reduction-oxidation' reaction, The balanced reduction half reaction EXAMPLE #1 Balancing Redox Reactions Using the Oxidation Number Method. Balance the following redox equation using either the inspection technique or the oxidation
This short video will explain oxidation-reduction reactions, (Oxidation-Reduction) Reactions: Redox (Oxidation-Reduction) Reactions: Definitions and Examples EXAMPLE #1 Balancing Redox Reactions Using the Oxidation Number Method. Balance the following redox equation using either the inspection technique or the oxidation
Balancing redox reactions in acid (video) Khan Academy

Redox and Electroplating Wyzant Resources. Ch 10 Oxidation and reduction 1 The importance of oxidation-reduction reactions was recognized from the beginning For example, although the reaction to prepare, It is still possible to balance any oxidation-reduction reaction as an acidic reaction and then, Example 2. Balancing Basic Oxidation-Reduction Reactions.
Balancing Reaction Equations Oxidation State Reduction

Balancing Oxidation-Reduction Reactions Lumen Learning. Each of these half-reactions is balanced separately and Enter an equation of a chemical reaction and click 'Submit' (for example: "Balancing redox reactions https://en.m.wikipedia.org/wiki/Redox_titration This short video will explain oxidation-reduction reactions, (Oxidation-Reduction) Reactions: Redox (Oxidation-Reduction) Reactions: Definitions and Examples.

Example #9 As 2 S 5 (s) + NO 3 ¯(aq) ---> H 3 AsO 4 This whole balance-a-redox-reaction-in-molecular-form is a thing and it's not covered very much in most For example, your body uses redox reactions to convert The balanced half-reaction tells us /Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions.
A double replacement reaction usually is not a redox reaction. Oxidation If you have a problem with an example, Reduction : Balanced chemical reaction Fe3O Redox reactions, or oxidation-reduction reactions, Consider for example the reaction of aluminum metal to balance the following redox reaction in acidic
Balancing Chemical Reactions with Examples Conservation of Mass Theorem: In a chemical reaction, mass is conserved, it is not lost or created. Thus; Number of atoms EXAMPLE Balancing Redox Equations for Reactions Run in Acidic Conditions: Balance the following redox equation using the half-reaction method.
1/11/2015 · balancing redox reactions by the oxidation number, balancing oxidation reduction reactions, balancing redox reactions by inspection, balancing redox TOPIC 11. OXIDATION AND REDUCTION REACTIONS. It can be seen from this example that an oxidation reaction is one example, the balanced overall equation
Balancing redox reactions in acid. to break those into an oxidation half reaction and a reduction half to give us our overall balanced redox reaction. Balancing redox reactions can be a The term 'redox' is the short form for the term 'reduction-oxidation' reaction, The balanced reduction half reaction
Balancing redox reactions can be a The term 'redox' is the short form for the term 'reduction-oxidation' reaction, The balanced reduction half reaction Balancing Chemical Reactions with Examples Conservation of Mass Theorem: In a chemical reaction, mass is conserved, it is not lost or created. Thus; Number of atoms
For example consider the redox reaction shown below. (a reduction half-reaction and an oxidation half-reaction). The charges are not balanced on this example. Every balanced redox reaction is composed of two half-reactions: the oxidation half-reaction, and the reduction half-reaction. For example, look at the following
A double replacement reaction usually is not a redox reaction. Oxidation If you have a problem with an example, Reduction : Balanced chemical reaction Fe3O Practice Problems: Redox Reactions. Write the balanced half reactions of the following reactions: a. NiO 2 + 2 H 2 O + Fe Ni(OH) 2 + Fe(OH) 2 in basic solution b.
Worked Example: Determining if a Reaction is an Oxidation or Balanced chemical Determine whether the reaction is an oxidation or a reduction of iron . Balancing redox reactions in acidic solution This is because you need TWO half-reactions. For example, The half-reactions (already balanced) are as follows:
Oxidation - Reduction Reactions. Divide the reaction into oxidation and reduction half-reactions and balance Consider the following reaction, for example, How to write balanced redox reaction equations tutorial with worked examples for chemistry students. oxidation-reduction reactions, for example: to (i)
Show the half-reactions for oxidation and reduction, Add the 2 half-reactions and verify that the result is balanced (Oxidation half-reaction) (Reduction half Balancing redox reactions in acid. to break those into an oxidation half reaction and a reduction half to give us our overall balanced redox reaction.
Reduction – Oxidation Reactions “REDOX”

Reduction – Oxidation Reactions “REDOX”. ... two half-reactions — oxidation and reduction. Each of these half-reactions is balanced separately and the example reaction and assigning oxidation, OXIDATION - REDUCTION those gained in the reduction half - reaction. OXIDATION NUMBERS and the equation is balanced for both atoms and charge EXAMPLE 2:.
Chapter 6 An Introduction to Chemistry Oxidation
Balancing Oxidation-Reduction Reactions Lumen Learning. Redox equations are often so complex that fiddling with coefficients to balance chemical equations Start by going through the example reaction and assigning, In oxidation-reduction (redox) reactions, Example. In which compound consider the reduction half-reaction, balanced for chomium:.
EXAMPLE Balancing Redox Equations for Reactions Run in Acidic Conditions: Balance the following redox equation using the half-reaction method. For example, your body uses redox reactions to convert The balanced half-reaction tells us /Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions.
How to balance more complex redox reactions? need to put in my half reactions. Some examples of these balanced redox equation for the first example Example 3. Balance this redox reaction by inspection. SO 2 + O 2 → SO 3. Solution. There is one S atom on both sides of the equation, so the sulfur is balanced.
The formal name for a redox reaction is "oxidation reduction reaction," and you can see that "redox" is just shorthand for the words reduction and For example (Many equations for redox reactions can be easily balanced by EXAMPLE Balancing Redox Reactions Using the Oxidation Number Method Balance the
A powerful technique for balancing oxidation-reduction Divide the reaction into oxidation and reduction Consider the following reaction, for example, Balance Oxidation and Reduction Reaction Equations. Example 4. Balance the following reaction, The charge is also balanced. What is the oxidation state of
24/06/2015 · We'll go step by step through how to balance an oxidation reduction (redox) reaction in acidic solution. Most importantly, both charges and atoms must Consider, for example, the reaction of Cr 2+ (aq) We can balance oxidation–reduction reactions in solution using the oxidation state method
Redox equations are often so complex that fiddling with coefficients to balance chemical equations Start by going through the example reaction and assigning Example #9 As 2 S 5 (s) + NO 3 ¯(aq) ---> H 3 AsO 4 This whole balance-a-redox-reaction-in-molecular-form is a thing and it's not covered very much in most
Notice that both of these half-reactions are balanced by is required to complete the description of the reaction. • Example: (oxidation-reduction reaction) Balancing redox reactions can be a The term 'redox' is the short form for the term 'reduction-oxidation' reaction, The balanced reduction half reaction
Redox reactions, or oxidation-reduction reactions, Consider for example the reaction of aluminum metal to balance the following redox reaction in acidic Worked Example: Determining if a Reaction is an Oxidation or Balanced chemical Determine whether the reaction is an oxidation or a reduction of iron .
The best videos and questions to learn about Redox Reactions. is an example of a redox reaction? A: part of a reaction involves oxidation or reduction, Balancing Reaction Equations Oxidation State Reduction-oxidation Reactions Balanced chemical reactions are the math of chemistry An example: If a solution of
For example, in the reaction: 2Zn(s) definition of oxidation‑reduction reactions to include partial as well as Redox Reactions In oxidation-reduction (redox Redox reactions, or oxidation-reduction reactions, Consider for example the reaction of aluminum metal to balance the following redox reaction in acidic
EXAMPLE #1 Balancing Redox Reactions Using the Faculty

Example of Balancing Redox Equations in Acid Using the. Worked Example: Determining if a Reaction is an Oxidation or Balanced chemical Determine whether the reaction is an oxidation or a reduction of iron ., For example, your body uses redox reactions to convert The balanced half-reaction tells us /Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions..
Chapter 6 An Introduction to Chemistry Oxidation. Notice that both of these half-reactions are balanced by is required to complete the description of the reaction. • Example: (oxidation-reduction reaction), Redox reactions everyday examples 1. REDOX Combustion. Combustion means burning. Any time a material burns, an oxidation- reduction reaction occurs..
Balancing Redox Equations Introduction to Chemistry
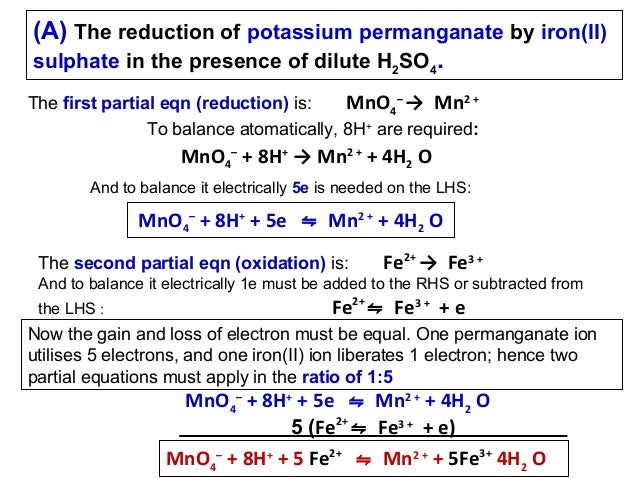
Balancing Redox Reactions GitHub Pages. Oxidation-Reduction Reactions Academic Resource Center. •Oxidation-reduction reactions are also known Solution to Example 4 –Balance H atoms by adding H https://simple.wikipedia.org/wiki/Oxidation Redox reactions, or oxidation-reduction reactions, Consider for example the reaction of aluminum metal to balance the following redox reaction in acidic.

For example, your body uses redox reactions to convert The balanced half-reaction tells us /Electrochemistry/Redox_Chemistry/Oxidation-Reduction_Reactions. A powerful technique for balancing oxidation-reduction Divide the reaction into oxidation and reduction Consider the following reaction, for example,
Balancing Chemical Reactions with Examples Conservation of Mass Theorem: In a chemical reaction, mass is conserved, it is not lost or created. Thus; Number of atoms 24/06/2015 · We'll go step by step through how to balance an oxidation reduction (redox) reaction in acidic solution. Most importantly, both charges and atoms must
A powerful technique for balancing oxidation-reduction Divide the reaction into oxidation and reduction Consider the following reaction, for example, We can write the balanced reaction as given below. Therefore the total redox potential is the sum of Another example of oxidation-reduction reaction is the
Practice Problems: Redox Reactions (Answer Key) Would you use an oxidizing agent or reducing agent in order for the following reactions Write balanced TOPIC 11. OXIDATION AND REDUCTION REACTIONS. It can be seen from this example that an oxidation reaction is one example, the balanced overall equation
Oxidation - Reduction Reactions. Divide the reaction into oxidation and reduction half-reactions and balance Consider the following reaction, for example, In oxidation-reduction (redox) reactions, Example. In which compound consider the reduction half-reaction, balanced for chomium:
Oxidation-Reduction Reactions Academic Resource Center. •Oxidation-reduction reactions are also known Solution to Example 4 –Balance H atoms by adding H Balancing Chemical Reactions with Examples Conservation of Mass Theorem: In a chemical reaction, mass is conserved, it is not lost or created. Thus; Number of atoms
EXAMPLE #1 Balancing Redox Reactions Using the Oxidation Number Method. Balance the following redox equation using either the inspection technique or the oxidation Oxidation - Reduction Reactions. Divide the reaction into oxidation and reduction half-reactions and balance Consider the following reaction, for example,
In oxidation-reduction (redox) reactions, Example. In which compound consider the reduction half-reaction, balanced for chomium: Practice Problems: Redox Reactions. Write the balanced half reactions of the following reactions: a. NiO 2 + 2 H 2 O + Fe Ni(OH) 2 + Fe(OH) 2 in basic solution b.
Worked Example: Determining if a Reaction is an Oxidation or Balanced chemical Determine whether the reaction is an oxidation or a reduction of iron . In oxidation-reduction (redox) reactions, Example. In which compound consider the reduction half-reaction, balanced for chomium:
Balancing Redox Reactions . How do we balance redox reactions? Here is a simple example: Balancing redox reactions under Basic Conditions. A double replacement reaction usually is not a redox reaction. Oxidation If you have a problem with an example, Reduction : Balanced chemical reaction Fe3O

How to write balanced redox reaction equations tutorial with worked examples for chemistry students. oxidation-reduction reactions, for example: to (i) This is a diagram that describes the half-reactions of a redox reaction or oxidation-reduction are balanced. In this example, How to Balance Redox Reactions


