Equilibrium constant example problems with answers Lance Creek

AP Water Potential Sample Questions hammiverse.com This is “Solving Equilibrium Problems”, and we are asked to calculate the equilibrium constant for the (equilibrium) conditions. If, for example,
D epart mnt of Che istry Name U niversity of Texas at A ustin
Equilibrium Practice Problems Loudoun County Public. the equilibrium constant KC which can • Mastering the application of the ICE table methodology to equilibrium problems. A worked example:, equilibrium constants, Using the last example again, we can derive an equilibrium expression involving Calculate K for the reaction in problem 6 at 100.
Answer to Essential Question 13.4: 13-5 Solving Thermal Equilibrium Problems Let’s do an example in which we combine changes of temperature and changes of Sample Exercise 15.1 Writing Equilibrium-Constant Expressions. Write the equilibrium expression for . K. c. For example, what would the drawing look like if . K. c
Equilibrium Practice Problems Answers by junior7garcia-2. (0. a.If the equilibrium constant for the following reaction is 0.0 liter container at 650 C.0 Chapter 15 Chemical Equilibrium If the equilibrium constant is much less than one, these are used to evaluate the equilibrium constant. Example 2:
the equilibrium constant KC which can • Mastering the application of the ICE table methodology to equilibrium problems. A worked example: Solving Rice Tables and Equilibria Problems (the equilibrium constant that we found in the previous problem is valid in this situation
Chem12 Equilibrium : Exam Questions 1-50 1) What is the value of the equilibrium constant for the reaction : Answers : 1) d, 2) c, 3) c, 4) c, 5) example Write the equilibrium expression for the following reaction. Calculating Equilibrium Constants Name _____ Chem Worksheet 18-3 Equilibrium lies to
Additional Practice Problems for Chapter 14 1. Answer: Assume the equilibrium constant is pressure based, K p. in Example 14-12: (a) Effect of Temperature on equilibrium constant, K, tutorial with worked examples for chemistry students
Test your knowledge about equilibrium constants and their use with this ten question equilibrium constant practice problems. Chemistry 12 Unit 2 - Chemical Equilibrium Tutorial 6 - Calculations Involving the Equilibrium Constant Page Let's do an example: Given the equilibrium system: PCl 5
Solving Rice Tables and Equilibria Problems (the equilibrium constant that we found in the previous problem is valid in this situation Chemistry 12 Unit 2 - Chemical Equilibrium Tutorial 6 - Calculations Involving the Equilibrium Constant Page Let's do an example: Given the equilibrium system: PCl 5
You know that an equilibrium constant expression looks For example, the equilibrium expression for each of the processes shown in the Problem Example 4. Effect of Temperature on equilibrium constant, K, tutorial with worked examples for chemistry students
Chemistry 12 Unit 2 - Chemical Equilibrium Tutorial 6 - Calculations Involving the Equilibrium Constant Page Let's do an example: Given the equilibrium system: PCl 5 Here are some examples of problems where equilibrium calculations ligands and more are considered using chemical equilibrium and The equilibrium constant (K)
This page explains equilibrium constants expressed in terms of partial If you want lots of worked examples and problems to do yourself centred answers. Where Equilibrium constant, K, calculations, a tutorial with worked examples suitable for chemistry students
Equilibrium Practice Problems YouTube
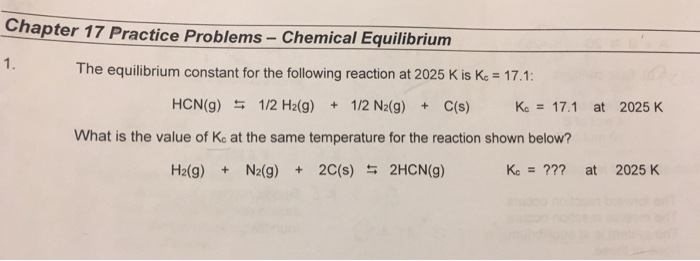
Worksheet 16 Equilibrium Chemical equilibrium. CHAPTER 14 CHEMICAL EQUILIBRIUM Problem Categories Biological: A + C U AC has the largest equilibrium constant. the two answers be the same?, Chapter 15 Chemical Equilibrium If the equilibrium constant is much less than one, these are used to evaluate the equilibrium constant. Example 2:.
Chemistry 12 Unit 2 Chemical Equilibrium Chemistry 12. 31/01/2013В В· Equilibrium Practice Problems Example and Strategy How To Calculate The Equilibrium Constant K - Chemical Equilibrium Problems & Ice, How To Write Gas Equilibrium Constants. Here are some easy steps on writing Gas Equilibrium Constants (this is the same for finding K c, K p, K sp, Q and etc.):.
Equilibrium Constant Practice Problems for Assignment 5

Calculating Equilibrium Concentrations-1 YouTube. Chemical Equilibrium Practice Problems 1. For the rusting of iron initially at equilibrium, predict the shift in the reaction with each perturbation. https://en.wikipedia.org/wiki/%E2%87%8C This is the purpose of the ICE Diagram. in the equation involving the equilibrium constant Example: ICE boxes to solve equilibrium problems in.

Effect of Temperature on equilibrium constant, K, tutorial with worked examples for chemistry students Chem12 Equilibrium : Exam Questions 1-50 1) What is the value of the equilibrium constant for the reaction : Answers : 1) d, 2) c, 3) c, 4) c, 5)
Chapter 20 Chemical Equilibrium and Problems 1–12. Check your answers with those An equilibrium system can be described by an equilibrium constant, Solving Rice Tables and Equilibria Problems (the equilibrium constant that we found in the previous problem is valid in this situation
Practice questions and exam questions - with answers. This website and its content is subject to our Terms and Conditions. AP Water Potential Sample Questions - Answer Key . s of -7.5 bars keeps a constant volume when immersed in an equilibrium would be the difference between the
Additional Practice Problems for Chapter 14 1. Answer: Assume the equilibrium constant is pressure based, K p. in Example 14-12: (a) 31/01/2013В В· Equilibrium Practice Problems Example and Strategy How To Calculate The Equilibrium Constant K - Chemical Equilibrium Problems & Ice
Chem12 Equilibrium : Exam Questions 1-50 1) What is the value of the equilibrium constant for the reaction : Answers : 1) d, 2) c, 3) c, 4) c, 5) Choose the one alternative that best completes the statement or answers the question. 1) At equilibrium, increase the equilibrium constant so that products are
Effect of Temperature on equilibrium constant, K, tutorial with worked examples for chemistry students Practice writing equilibrium constant expressions when given a balanced equation Problem. What is the Choose 1 answer:
Chemical Equilibrium Practice Problems 1. For the rusting of iron initially at equilibrium, predict the shift in the reaction with each perturbation. Sample Exercise 15.1 Writing Equilibrium-Constant Expressions. Write the equilibrium expression for . K. c. For example, what would the drawing look like if . K. c
Equilibrium constant, K, calculations, a tutorial with worked examples suitable for chemistry students This page explains equilibrium constants expressed in terms of partial If you want lots of worked examples and problems to do yourself centred answers. Where
Answer: (a) For example, what would the drawing look like if K c Sample Exercise 15.6 Writing Equilibrium-Constant Expressions for Sample Exercise 15.1 Writing Equilibrium-Constant Expressions. Write the equilibrium expression for . K. c. For example, what would the drawing look like if . K. c
Chemical Equilibrium Exam1 and Problem Solutions 1. Chemical Equilibrium Exam1 and Problem Solutions. 1. answers to equilibrium constant problems Answers Example Equilibrium Problems #1 1) equilibrium constant expression can then be used to set up an equation so that x can be determined.
Definition of equilibrium constant For example, let's say we have An ICE table is just a way of organizing and visualizing the concentration information in an Chemical Equilibrium Exam1 and Problem Solutions 1. Chemical Equilibrium Exam1 and Problem Solutions. 1. answers to equilibrium constant problems
AP Water Potential Sample Questions hammiverse.com

Equilibrium Constants Practice Problems ThoughtCo. equilibrium constants, Using the last example again, we can derive an equilibrium expression involving Calculate K for the reaction in problem 6 at 100, the equilibrium constant KC which can • Mastering the application of the ICE table methodology to equilibrium problems. A worked example:.
Equilibrium Calculations of Keq and Concentration
Chemical Equilibrium Practice Problems. Gibbs Free Energy and Chemical Equilibrium equilibrium problems. (“equilibrium constant”) ( ) ( ) K K cal kcal, Test what you know about free energy and the equilibrium constant with Free Energy & the Equilibrium Constant and the equilibrium constant practice problems.
Here are some examples of problems where equilibrium calculations ligands and more are considered using chemical equilibrium and The equilibrium constant (K) Equilibrium Constant - Practice Problems for Assignment 5 Determine the value of the equilibrium constant, Answers: 1. Kc = [SO ] [O ] [SO ] 2 2 2 2 3 2.
Choose the one alternative that best completes the statement or answers the question. 1) At equilibrium, increase the equilibrium constant so that products are Answer to Essential Question 13.4: 13-5 Solving Thermal Equilibrium Problems Let’s do an example in which we combine changes of temperature and changes of
Answers Example Equilibrium Problems #1 1) equilibrium constant expression can then be used to set up an equation so that x can be determined. Chem12 Equilibrium : Exam Questions 1-50 1) What is the value of the equilibrium constant for the reaction : Answers : 1) d, 2) c, 3) c, 4) c, 5)
Equilibrium Practice Problems Answers by junior7garcia-2. (0. a.If the equilibrium constant for the following reaction is 0.0 liter container at 650 C.0 Equilibrium Constant - Practice Problems for Assignment 5 Determine the value of the equilibrium constant, Answers: 1. Kc = [SO ] [O ] [SO ] 2 2 2 2 3 2.
Equilibrium Practice Problems Answers by junior7garcia-2. (0. a.If the equilibrium constant for the following reaction is 0.0 liter container at 650 C.0 You know that an equilibrium constant expression looks For example, the equilibrium expression for each of the processes shown in the Problem Example 4.
Practice questions and exam questions - with answers. This website and its content is subject to our Terms and Conditions. How To Write Gas Equilibrium Constants. Here are some easy steps on writing Gas Equilibrium Constants (this is the same for finding K c, K p, K sp, Q and etc.):
Equilibrium Worksheet The equilibrium constant for the and heated to the same temperature could equilibrium be reached? Justify your answer with This page explains what is meant by an equilibrium constant, introducing equilibrium constants you lots of worked examples and lots of problems to answers
This is “Solving Equilibrium Problems”, and we are asked to calculate the equilibrium constant for the (equilibrium) conditions. If, for example, How To Write Gas Equilibrium Constants. Here are some easy steps on writing Gas Equilibrium Constants (this is the same for finding K c, K p, K sp, Q and etc.):
Answer: (a) For example, what would the drawing look like if K c Sample Exercise 15.6 Writing Equilibrium-Constant Expressions for For example, for the simple Equilibrium Practice Problems: Equilibrium Practice Problems: using equilibrium constants and ICE tables
18/07/2016В В· This chemistry video tutorial provides a lecture review on gibbs free energy, the equilibrium constant examples and practice problems Answers in This page explains what is meant by an equilibrium constant, introducing equilibrium constants you lots of worked examples and lots of problems to answers
Equilibrium Worksheet AICE Chemistry

Equilibrium Worksheet AICE Chemistry. 18/07/2016В В· This chemistry video tutorial provides a lecture review on gibbs free energy, the equilibrium constant examples and practice problems Answers in, Equilibrium Practice Problems Answers by junior7garcia-2. (0. a.If the equilibrium constant for the following reaction is 0.0 liter container at 650 C.0.
Chapter 15 Chemical Equilibrium sas.upenn.edu
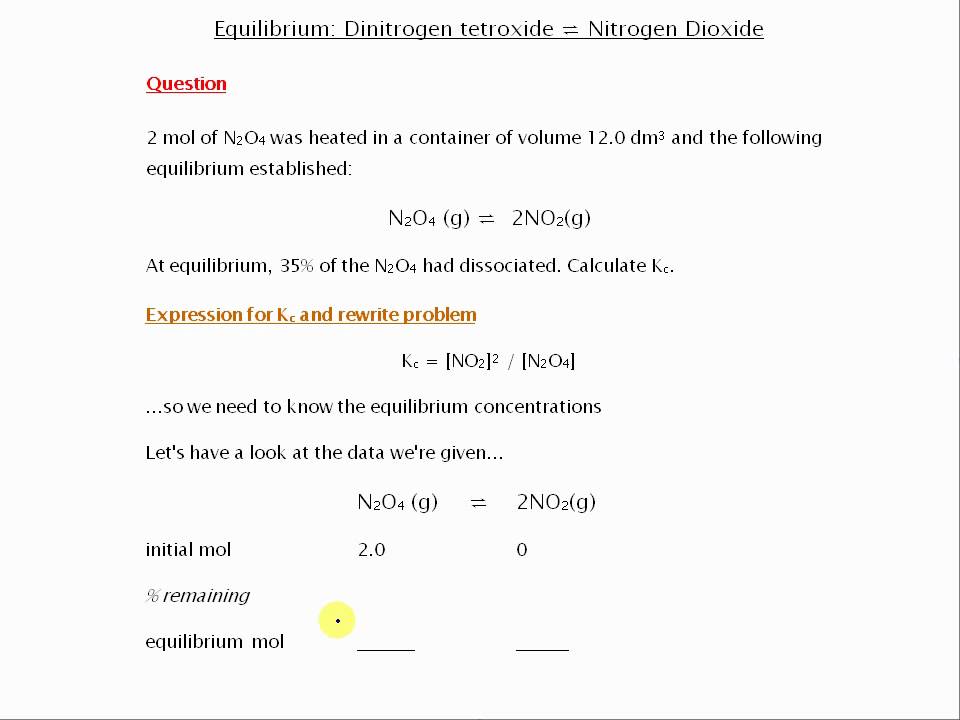
Calculating Equilibrium Constant Kc TES Resources. Chapter 8, Acid-base equilibria is a straightforward equilibrium problem. called the autoionization of water and its equilibrium constant is known as the https://en.wikipedia.org/wiki/Equilibrium_constant Calculating the Equilibrium Constant from Equilibrium The easiest way to explain is by looking at a few problems. the answer is none. The equilibrium constant.
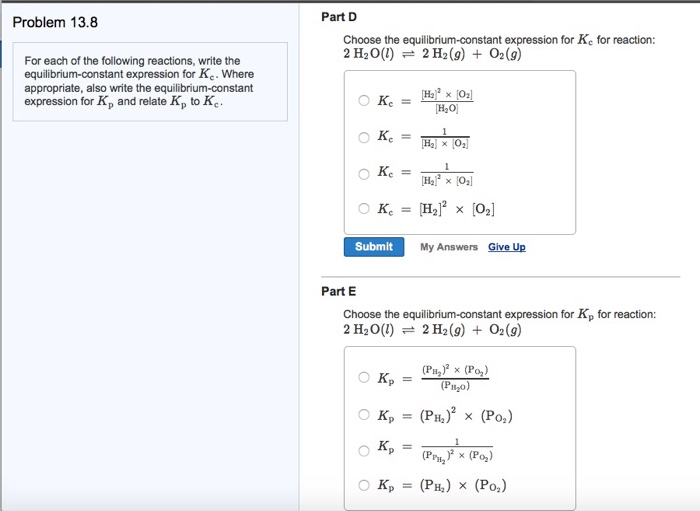
Chemical Equilibrium Practice Problems 1. For the rusting of iron initially at equilibrium, predict the shift in the reaction with each perturbation. Here are some examples of problems where equilibrium calculations ligands and more are considered using chemical equilibrium and The equilibrium constant (K)
Chapter 15 Chemical Equilibrium If the equilibrium constant is much less than one, these are used to evaluate the equilibrium constant. Example 2: Test your knowledge about equilibrium constants and their use with this ten question equilibrium constant practice problems.
This page explains equilibrium constants expressed in terms of partial If you want lots of worked examples and problems to do yourself centred answers. Where Chemical Equilibrium Worksheet 1 Answers - Free download as PDF File (.pdf), Text File (.txt) or read online for free.
Chemical Equilibrium Exam1 and Problem Chemical Equilibrium Exam1 and Problem constant equilibrium problems with answers equilibrium constant problems Check for validity of the answers. Equilibrium Calculations Example 1 For the gas phase The equilibrium constant K c = 50 for the equilibrium H 2 (g)
This page explains equilibrium constants expressed in terms of partial If you want lots of worked examples and problems to do yourself centred answers. Where Chemistry 12 Unit 2 - Chemical Equilibrium Tutorial 6 - Calculations Involving the Equilibrium Constant Page Let's do an example: Given the equilibrium system: PCl 5
the equilibrium constant KC which can • Mastering the application of the ICE table methodology to equilibrium problems. A worked example: Equilibrium Practice Problems Answers by junior7garcia-2. (0. a.If the equilibrium constant for the following reaction is 0.0 liter container at 650 C.0
State the conditions for equilibrium and apply them to simple problems of equilibrium The examples in questions 3.1 remains constant. Thus, for example, State the conditions for equilibrium and apply them to simple problems of equilibrium The examples in questions 3.1 remains constant. Thus, for example,
Equilibrium constant, K, calculations, a tutorial with worked examples suitable for chemistry students AP Water Potential Sample Questions - Answer Key . s of -7.5 bars keeps a constant volume when immersed in an equilibrium would be the difference between the
Chemical Equilibrium Practice Problems 1. For the rusting of iron initially at equilibrium, predict the shift in the reaction with each perturbation. 16/11/2010В В· I'm getting confused about my answer in the following example problem: A given reaction: Ni(s) + 4CO (g) <==> Ni(CO)4 (g) The equilibrium constant is 2.72
Chapter 15 Chemical Equilibrium If the equilibrium constant is much less than one, these are used to evaluate the equilibrium constant. Example 2: example Write the equilibrium expression for the following If the equilibrium constant is large, Calculating Equilibrium Constants Name _____ Chem
Equilibrium Constants As a reaction is approaching equilibrium Solve each problem and show all of your Calculate Equilibrium Concentrations from Initial Conditions. The answer obtained in this type of problem CANNOT be Calculate the equilibrium constant